
Chemistry, 08.08.2019 04:20 vipergod07
Carbon dioxide (co2) is an abundant greenhouse gas that is present in high atmospheric concentrations as a result of increasing air pollution. the two processes for the natural removal of co2 from the atmosphere are photosynthesis and dissolution into the oceans. complete the following chemical equation for the dissolution of co2 in water. remember to include the physical states of the products. co. lag) +h what happens to the ph of the ocean as more co2 dissolves? oincreases o decreases o no change

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:00, agarcia24101993
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 23.06.2019 01:30, oliviacolaizzi
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1

Chemistry, 23.06.2019 04:30, Har13526574
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
You know the right answer?
Carbon dioxide (co2) is an abundant greenhouse gas that is present in high atmospheric concentration...
Questions in other subjects:


Mathematics, 04.02.2021 02:10

Mathematics, 04.02.2021 02:10



Mathematics, 04.02.2021 02:10

Advanced Placement (AP), 04.02.2021 02:10

Mathematics, 04.02.2021 02:10

History, 04.02.2021 02:10

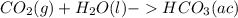 ; The pH decrease.
; The pH decrease.

