
Chemistry, 08.08.2019 00:20 aroland1990x
Calculate the δ h°rxn for the decomposition of calcium carbonate to calcium oxide and carbon dioxide. δ h°f [caco3(s)] = –1206.9 kj/mol; δ h°f [cao(s)] = –635.1 kj/mol; δ h°f [co2(g)] = –393.5 kj/mol

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1


Chemistry, 22.06.2019 13:30, yasiroarafat12
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
Calculate the δ h°rxn for the decomposition of calcium carbonate to calcium oxide and carbon dioxide...
Questions in other subjects:




Biology, 07.09.2021 20:40

Mathematics, 07.09.2021 20:40

Mathematics, 07.09.2021 20:40

Mathematics, 07.09.2021 20:40


Mathematics, 07.09.2021 20:40

Biology, 07.09.2021 20:40


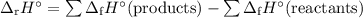
![\begin{array}{rcl}\Delta_{\text{r}}H^{\circ}& = & [(-635.1 + (-393.5)] - (-1206.9)\\& = & -1028.6 +1206.9\\& = & \mathbf{178.3}\\\end{array}\\\text{The enthalpy of decomposition is } \boxed{\textbf{178.3 kJ/mol}}](/tpl/images/0172/9753/c40b5.png) l}}
l}}

