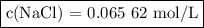Chemistry, 07.08.2019 01:10 BrainlyAvenger
To study the effect of dissolved salt on the rusting of an iron sample, a student prepared a solution of nacl by dissolving 3.038 g of nacl in enough
water to make 792.2 ml of solution. what is the molarity of this solution?
c(nacl)=

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Cooldude3966
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2

Chemistry, 21.06.2019 21:00, alexmarche4675
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 02:30, ulilliareinhart2
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2
You know the right answer?
To study the effect of dissolved salt on the rusting of an iron sample, a student prepared a solutio...
Questions in other subjects:







Arts, 22.01.2021 23:00


English, 22.01.2021 23:00