
At 700 k, the reaction2so2(g) + o2(g) 2so3(g)has the equilibrium constant kc = 4.3 x 106, and the following concentrations are present: [so2] = 0.010 m; [so3] = 10.m; [o2] = 0.010 m. is the mixture at equilibrium? if not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium? yes, the mixture is at equilibrium. no, left to right. no, right to left. there is not enough information to be able to predict the direction.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
At 700 k, the reaction2so2(g) + o2(g) 2so3(g)has the equilibrium constant kc = 4.3 x 106, and the fo...
Questions in other subjects:


English, 18.10.2020 14:01


History, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01

Chemistry, 18.10.2020 14:01



Spanish, 18.10.2020 14:01

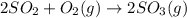
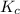 = 4.3 \times 10^{6}[/tex].
= 4.3 \times 10^{6}[/tex].![[SO_2]](/tpl/images/0172/4735/0303a.png) = 0.010 M;
= 0.010 M; ![[SO_3]](/tpl/images/0172/4735/8a06f.png) = 10.M;
= 10.M; ![[O_2]](/tpl/images/0172/4735/b0db0.png) = 0.010 M.
= 0.010 M.![\frac{[SO_{3}]^{2}}{[SO_{2}]^{2}[O_{2}]}](/tpl/images/0172/4735/2750c.png)
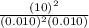
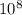
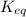 , then reaction moves in the backward direction.
, then reaction moves in the backward direction.

