
Which of the following statements is/are correct? 1. for a chemical system, if the reaction quotient (q) is greater than k, reactant must be converted to products to reach equilibrium.2. for a chemical system at equilibrium, the forward and reverse rates of reaction are equal.3. for a chemical system at equilibrium, the concentrations of products divided by the concentrations of reactants equals one.1 only2 only3 only1 and 21, 2, and 3

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2



Chemistry, 23.06.2019 13:00, kayleegeise
Write the balanced chemical reaction for the formation of fe2(so4)3 from fe2o3 and so3 and determine how many moles of fe2(so4)3 are formed when 12.7 mol of so3 are reacted.
Answers: 1
You know the right answer?
Which of the following statements is/are correct? 1. for a chemical system, if the reaction quotient...
Questions in other subjects:




Mathematics, 15.01.2021 21:10

Business, 15.01.2021 21:10

Mathematics, 15.01.2021 21:10

Mathematics, 15.01.2021 21:10

History, 15.01.2021 21:10


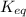 , then it means that the reaction is proceeding in the backward reaction. Whereas if Q <
, then it means that the reaction is proceeding in the backward reaction. Whereas if Q < 

