
Which reactant, and how many grams of it, is left over after 16.0 g of mno2 (fw = 86.9 g/mol) and 30.0 g of hcl (fw = 36.5 g/mol) react according to the following chemical equation? mno2 + 4 hcl ® mncl2 + cl2 + 2 h2o3.1 g hcl23.3 g hcl4.02 g mno28.0 g mno212.1 g mno2

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, ksalinas7404
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 00:10, bossboybaker
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 01:00, bettybales1986
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
Which reactant, and how many grams of it, is left over after 16.0 g of mno2 (fw = 86.9 g/mol) and 30...
Questions in other subjects:




History, 16.10.2020 05:01


Mathematics, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01



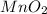 = 16.0 g
= 16.0 g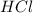 = 30.0 g
= 30.0 g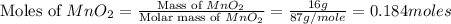
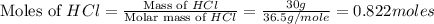
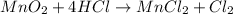
 moles of
moles of 



