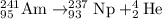Chemistry, 06.08.2019 04:20 fangirl2837
There are several technological applications for the transuranium elements (z > 92). an important one is in smoke detectors, which can use the decay of a tiny amount of americium-241 to neptunium-237. what subatomic particle is emitted from that decay process? explain your answer.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 13:00, netflixacc0107
Amixture with the same composition throughout is!
Answers: 1

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
There are several technological applications for the transuranium elements (z > 92). an importan...
Questions in other subjects:


Mathematics, 15.07.2020 02:01