
Chemistry, 06.08.2019 02:30 jayjay9434
The equilibrium constant kp for the reaction pcl5(g) ⇌ pcl3(g) + cl2(g) is 1.05 atm at 250ºc. the reaction starts with a mixture of pcl5, pcl3, and cl2 at pressures 0.177 atm, 0.223 atm, and 0.111 atm, respectively, at 250ºc. when the mixture comes to equilibrium at that temperature, which pressures will have decreased and which will have increased? explain why.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, cordovamaria22
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 18:00, LuvieAnn1886
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1
You know the right answer?
The equilibrium constant kp for the reaction pcl5(g) ⇌ pcl3(g) + cl2(g) is 1.05 atm at 250ºc. the re...
Questions in other subjects:




Mathematics, 29.03.2021 19:50

Mathematics, 29.03.2021 19:50



Mathematics, 29.03.2021 19:50


History, 29.03.2021 19:50


 ,
,  , and
, and  at pressures as 0.177 atm, 0.223 atm, and 0.111 atm, respectively.
at pressures as 0.177 atm, 0.223 atm, and 0.111 atm, respectively.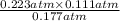
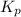 > 0.14 atm. As calculated value is less than the given value of
> 0.14 atm. As calculated value is less than the given value of 

