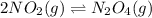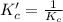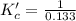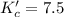The brown gas no2 and the colorless gas n2o4 exist in equilibrium,2no2 n2o4.in an experiment, 0.625 mole of n2o4 was introduced into a 5.00 l vessel and was allowed to decompose until equilibrium was reached. the concentration of n2o4 at equilibrium was 0.0750 m. calculate kc for the reaction.0.0500.07500.100.1257.5

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, slugmilk1090
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 00:00, chefdnguyen
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1


Chemistry, 22.06.2019 15:00, NatalieKnows
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
The brown gas no2 and the colorless gas n2o4 exist in equilibrium,2no2 n2o4.in an experiment, 0.625...
Questions in other subjects:



Social Studies, 27.02.2020 02:04




Chemistry, 27.02.2020 02:04



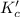 for the reaction is, 7.5
for the reaction is, 7.5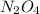 =
= 
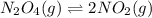
![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0172/0236/271f5.png)
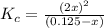
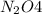 at equilibrium = 0.0750 M
at equilibrium = 0.0750 M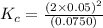
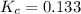
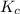 for the given reaction.
for the given reaction.