

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
You know the right answer?
What is the root-mean-square speed of chlorine gas molecules at a temperature of 346 k? (r = 8.31 j...
Questions in other subjects:

History, 05.05.2020 03:05




History, 05.05.2020 03:05

Mathematics, 05.05.2020 03:05




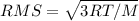
![RMS=\sqrt{3RT/M} \\RMS=\sqrt{[3\times8.314\times346]/71} \\RMS=\sqrt{121.54}\\RMS=11.02m/s](/tpl/images/0171/9917/00112.png)



