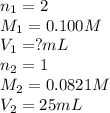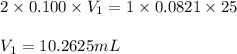Chemistry, 06.08.2019 01:30 heavendavis101
Sulfuric acid (h2so4) reacts with potassium hydroxide (koh) as follows. h2so4(aq) + 2 koh(aq) k2so4(aq) + 2 h2o(l) calculate the volume of 0.100 m sulfuric acid required to neutralize 25.0 ml of 0.0821 m koh.

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 05:00, shealynh52
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2
You know the right answer?
Sulfuric acid (h2so4) reacts with potassium hydroxide (koh) as follows. h2so4(aq) + 2 koh(aq) k2so4(...
Questions in other subjects:

Physics, 26.02.2020 00:33

Chemistry, 26.02.2020 00:33

Biology, 26.02.2020 00:33

Chemistry, 26.02.2020 00:33






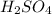 comes out to be 10.2625 mL.
comes out to be 10.2625 mL.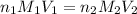
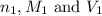 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 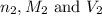 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.