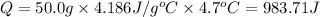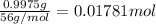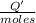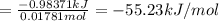Chemistry, 06.08.2019 01:20 larissacrystalow8g2w
0.9775 grams of an unknown compound is dissolved in 50.0 ml of water. initially the water temperature is 22.3 degrees celsius. after addition of the solid, the solution temperature is raised to about 27.0 degrees celsius. the substance is known to have a molar mass of about 56 g/mol. calculate the enthlapy of solution in kj/mol.

Answers: 3


Other questions on the subject: Chemistry



You know the right answer?
0.9775 grams of an unknown compound is dissolved in 50.0 ml of water. initially the water temperatur...
Questions in other subjects:


Chemistry, 10.07.2019 11:30

Biology, 10.07.2019 11:30

Mathematics, 10.07.2019 11:30

Chemistry, 10.07.2019 11:30

Mathematics, 10.07.2019 11:30

Chemistry, 10.07.2019 11:30



History, 10.07.2019 11:30