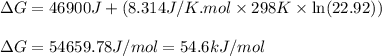Chemistry, 06.08.2019 01:10 candymorgan81
Consider the following reaction: co2(g)+ccl4(g)⇌2cocl2(g) calculate δg for this reaction at25 ∘c under these conditions: pco2pccl4pcocl2===0.140atm0.180atm0 .760atm δg∘f for co2(g) is −394.4kj/mol, δg∘f for ccl4(g) is −62.3kj/mol, and δg∘f for cocl2(g) is −204.9kj/mol. express the energy change in kilojoules per mole to one decimal place.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1


Chemistry, 22.06.2019 21:00, alwaysneedhelp84
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
Consider the following reaction: co2(g)+ccl4(g)⇌2cocl2(g) calculate δg for this reaction at25 ∘c un...
Questions in other subjects:

English, 30.12.2020 19:20

Chemistry, 30.12.2020 19:20







Mathematics, 30.12.2020 19:30

Chemistry, 30.12.2020 19:30

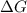 for the reaction is 54.6 kJ/mol
for the reaction is 54.6 kJ/mol 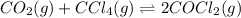
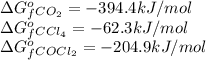
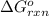 for the reaction, we use the equation:
for the reaction, we use the equation:![\Delta G^o_{rxn}=\sum [n\times \Delta G_f(product)]-\sum [n\times \Delta G_f(reactant)]](/tpl/images/0171/9686/1c133.png)
![\Delta G^o_{rxn}=[(2\times \Delta G^o_f_{(COCl_2)})]-[(1\times \Delta G^o_f_{(CO_2)})+(1\times \Delta G^o_f_{(CCl_4)})]](/tpl/images/0171/9686/08d2c.png)
![\Delta G^o_{rxn}=[(2\times (-204.9))-((1\times (-394.4))+(1\times (-62.3)))]\\\Delta G^o_{rxn}=46.9kJ=46900J](/tpl/images/0171/9686/b07a7.png)
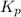 for the given reaction:
for the given reaction: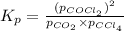
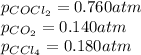
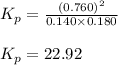
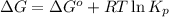
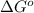 = Standard gibbs' free energy change of the reaction = 46900 J
= Standard gibbs' free energy change of the reaction = 46900 J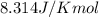
![25^oC=[25+273]K=298K](/tpl/images/0171/9686/df1f6.png)