
Chemistry, 06.08.2019 00:30 tybreyonnaHco7855
In the haber process for ammonia synthesis, k " 0.036 for n 2 (g) ! 3 h 2 (g) ∆ 2 nh 3 (g) at 500. k. if a 2.0-l reactor is charged with 1.42 bar of n 2 and 2.87 bar of h 2 , what will the equilibrium partial pressures in the mixture be?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, kev71
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1



Chemistry, 23.06.2019 01:00, only1cache
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2
You know the right answer?
In the haber process for ammonia synthesis, k " 0.036 for n 2 (g) ! 3 h 2 (g) ∆ 2 nh 3 (g) at 500....
Questions in other subjects:



English, 31.08.2019 00:00



Social Studies, 31.08.2019 00:00


History, 31.08.2019 00:00


Biology, 31.08.2019 00:00

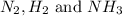 at equilibrium are, 1.133, 2.009, 0.574 bar respectively. The total pressure at equilibrium is, 3.716 bar
at equilibrium are, 1.133, 2.009, 0.574 bar respectively. The total pressure at equilibrium is, 3.716 bar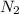 = 1.42 bar
= 1.42 bar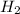 = 2.87 bar
= 2.87 bar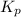 = 0.036
= 0.036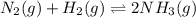
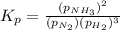
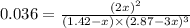
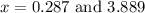
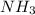 at equilibrium = 2x = 2 × 0.287 = 0.574 bar
at equilibrium = 2x = 2 × 0.287 = 0.574 bar

