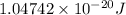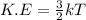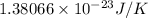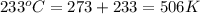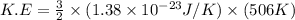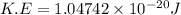Chemistry, 06.08.2019 00:20 kaleahearly123
Acylinder contains a mixture of helium and argon gas in equilibrium at a temperature of 233◦ c. the value of boltzmann’s constant is 1.38066 × 10−23 j/k, and avogadro’s number is 6.02 × 1023 mol−1. what is the average kinetic energy of each type of molecule? answer in units of j.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, tchase0616
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
You know the right answer?
Acylinder contains a mixture of helium and argon gas in equilibrium at a temperature of 233◦ c. the...
Questions in other subjects:

Mathematics, 04.08.2019 18:00

Mathematics, 04.08.2019 18:00


Mathematics, 04.08.2019 18:00

Mathematics, 04.08.2019 18:00

Mathematics, 04.08.2019 18:00

Spanish, 04.08.2019 18:00

Mathematics, 04.08.2019 18:00

Chemistry, 04.08.2019 18:00