
Chemistry, 06.08.2019 00:10 cthompson1107
How would the calculated value of ksp be affected by the accidental and unknowing collection of solid lead(ii) chloride via pipet in the gibb’s free energy experiment? this would not affect the calculated value of ksp. the calculated value of ksp would be too small. the calculated value of ksp would be too big.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, alexabdercmur
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 23.06.2019 07:30, jonquil201
Using this reversible reaction, answer the questions below: n2o4 2no2 (colorless) (reddish-brown) -as the temperature increased, what happened to the n2o4 concentration? -was the formation of reactants or products favored by the addition of heat? -which reaction is exothermic? right to left or left to right? -if the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct? n2o4 2no2 + 14 kcal n2o4 2no2, hr = +14 kcal n2o4 + 14 kcal 2no2 n2o4 2no2, hr = -14 kcal
Answers: 1

Chemistry, 23.06.2019 08:20, debramknoxx
At which temperature would a reaction with ah= -220 kj/mol and as=-0.05 kj/(mol-k) be spontaneous?
Answers: 2
You know the right answer?
How would the calculated value of ksp be affected by the accidental and unknowing collection of soli...
Questions in other subjects:


History, 08.12.2020 21:10


Mathematics, 08.12.2020 21:10


Mathematics, 08.12.2020 21:10

Medicine, 08.12.2020 21:10

Mathematics, 08.12.2020 21:10


English, 08.12.2020 21:10

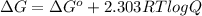 ..... (1)
..... (1)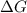 = 0. Also, Q = K this means that reaction is at equilibrium.
= 0. Also, Q = K this means that reaction is at equilibrium.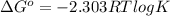 ....... (2)
....... (2)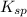
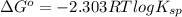 ....... (3)
....... (3)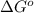 according to equation (3).
according to equation (3).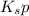 would be too big.
would be too big.

