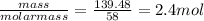Chemistry, 05.08.2019 22:30 izzynikkie
Consider the thermochemical equation for the combustion of acetone (c3h6o), the main ingredient in nail polish remover: c3h6o(l)+4 o2(g)→3 co2(g)+3 h2o(g)δh°rxn=−1790 kj if a bottle of nail polish remover contains 177 ml of acetone, how much heat is released by its complete combustion? the density of acetone is 0.788 g/ml.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:10, gungamer720
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
Consider the thermochemical equation for the combustion of acetone (c3h6o), the main ingredient in n...
Questions in other subjects:


History, 09.09.2019 20:30

Mathematics, 09.09.2019 20:30


Mathematics, 09.09.2019 20:30


Geography, 09.09.2019 20:30

Mathematics, 09.09.2019 20:30