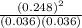Chemistry, 05.08.2019 21:20 ARAYAMYHAND
At a high temperature, equal concentrations of 0.160 mol/l of h2(g) and i2(g) are initially present in a flask. the h2 and i2 react according to the balanced equation below. h2(g) + i2(g) 2 hi(g)when equilibrium is reached, the concentration of h2(g) has decreased to 0.036 mol/l. what is the equilibrium constant, kc, for the reaction?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, tiwaribianca475
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2


Chemistry, 22.06.2019 23:30, Xavier8247
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
At a high temperature, equal concentrations of 0.160 mol/l of h2(g) and i2(g) are initially present...
Questions in other subjects:




English, 16.11.2020 01:00


English, 16.11.2020 01:00

Mathematics, 16.11.2020 01:00



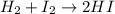
![[H_{2}]](/tpl/images/0171/8442/f6f78.png) = [0.160 - x] = 0.036 M
= [0.160 - x] = 0.036 M![[I_{2}]](/tpl/images/0171/8442/d0626.png) = [0.160 - x] = 0.036 M
= [0.160 - x] = 0.036 M 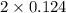
![K_{eq} = \frac{[HI]^{2}}{[H_{2}][I_{2}]}](/tpl/images/0171/8442/ed373.png)