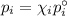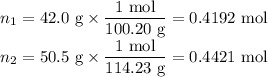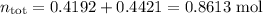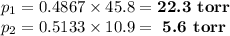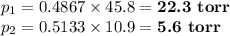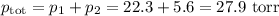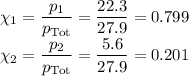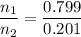Chemistry, 05.08.2019 21:20 DragonLovely
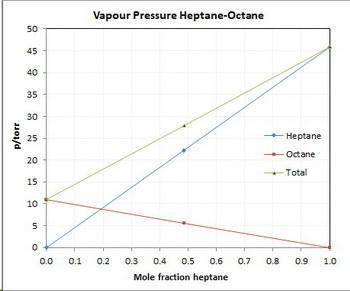
Asolution contains 42.0 g of heptane (c7h16) and 50.5 g of octane (c8h18) at 25 ∘c. the vapor pressures of pure heptane and pure octane at 25 ∘c are 45.8 torr and 10.9 torr, respectively. assuming ideal behavior, calculate each of the following. (note that the mole fraction of an individual gas component in an ideal gas mixture can be expressed in terms of the component's partial pressure.)a.) the vapor pressure of each of the solution components in the mixtureb.) the total pressure above the solutionc.) the composition of the vapor in mass percentd.) why is the composition of the vapor different from the composition of the solution?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, BigDough9090
Becquerel expected to find ( he developed the photographic plate that had sun-exposed minerals on top of it. becquerel expected to find ( he developed the photographic plate that had been in the closed drawer.
Answers: 2


Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

You know the right answer?
Asolution contains 42.0 g of heptane (c7h16) and 50.5 g of octane (c8h18) at 25 ∘c. the vapor pressu...
Questions in other subjects:





Mathematics, 12.10.2019 21:50



Social Studies, 12.10.2019 21:50


Social Studies, 12.10.2019 21:50