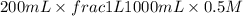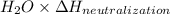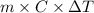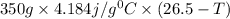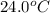Chemistry, 05.08.2019 19:10 camrenp9889
When 150. ml of 0.400 m h+ are mixed with 200. ml of 0.500 m oh-, the final temperature of the solution is 26.5°c. what was the initial temperature of the solution before the reaction occurred? assume that the solution has a total mass of 350. g and a specific heat capacity of 4.184 j/g°c. the enthalpy of neutralization for the reaction is -62.0 kj/mol of water produced.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 23.06.2019 05:20, cjking2320
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2

Chemistry, 23.06.2019 11:00, artiomtyler007
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was neutralized by 87 cm3 of 1.6 mol dm-3 naoh. the temperature rose from 298 k to 317.4 k. the specific heat capacity is the same as water, 4.18 j/k g. a. -101.37 kj b. 7055 kj c. 10,1365 kj
Answers: 1
You know the right answer?
When 150. ml of 0.400 m h+ are mixed with 200. ml of 0.500 m oh-, the final temperature of the solut...
Questions in other subjects:


SAT, 20.01.2022 01:40


Mathematics, 20.01.2022 01:40



Physics, 20.01.2022 01:40



SAT, 20.01.2022 01:40

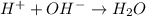
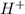 = volume × concentration of
= volume × concentration of 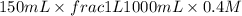
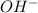 = volume × concentration of
= volume × concentration of