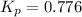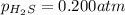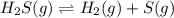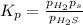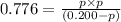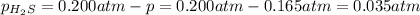Chemistry, 03.08.2019 03:30 kandikisses2101
At a certain temperature, the for the decomposition of h2s is 0.776. h2s(g)↽−−⇀h2(g)+s(g) initially, only h2s is present at a pressure of 0.200 atm in a closed container. what is the total pressure in the container at equilibrium?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 23.06.2019 06:00, asalimanoucha2v
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3

You know the right answer?
At a certain temperature, the for the decomposition of h2s is 0.776. h2s(g)↽−−⇀h2(g)+s(g) initially...
Questions in other subjects:

Mathematics, 20.11.2019 11:31

Mathematics, 20.11.2019 11:31





Mathematics, 20.11.2019 11:31



Mathematics, 20.11.2019 11:31