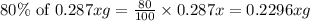Chemistry, 03.08.2019 00:30 trinigal83
The useful metal manganese can be extracted from the mineral rhodochrosite by a two-step in the first step, manganese(ii) carbonate and oxygen react to form manganese(iv) oxide and carbon dioxide: 2mnco3 + o2=2mno2 + 2co2in the second step, manganese(iv) oxide and aluminum react to form manganese and aluminum oxidide: 3mno2 + 4al = 3mn + 2al2o3 suppose the yield of the first step is 65.% and the yield of the second step is 80.%. calculate the mass of manganese(ii) carbonate required to make 8.0kg of manganese. be sure your answer has a unit symbol, if needed, and is rounded to 2 significant digits.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2
You know the right answer?
The useful metal manganese can be extracted from the mineral rhodochrosite by a two-step in the fir...
Questions in other subjects:




Mathematics, 02.04.2022 14:00



Mathematics, 02.04.2022 14:00



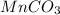 required are, 35 kg
required are, 35 kg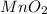 .
.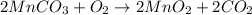
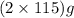 of
of 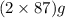 of
of 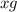 of
of 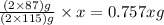 of
of 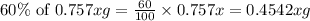
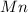 .
.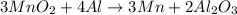
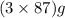 of
of 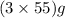 of
of 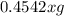 of
of 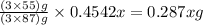 of
of