
Chemistry, 03.08.2019 00:10 babydoll1981
Carbon disulfide is a colorless liquid. when pure, it is nearly odorless, but the commercial product smells vile. carbon disulfide is used in the manufacture of rayon and cellophane. the liquid burns as follows: cs2(l) + 3o2(g) → co2(g) + 2so2(g)calculate the standard enthalpy change for this reaction usingstandard enthalpies of formation.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 04:00, Tiredd7838
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1

Chemistry, 23.06.2019 10:30, 7thaohstudent
What is the difference between skimming and absorbing methods of the oil removal
Answers: 2
You know the right answer?
Carbon disulfide is a colorless liquid. when pure, it is nearly odorless, but the commercial product...
Questions in other subjects:


Mathematics, 19.04.2021 04:30




Mathematics, 19.04.2021 04:30




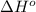
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0163/6329/45485.png)
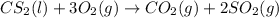
![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(CO_2)})+(2\times \Delta H^o_f_{(SO_2)})]-[(1\times \Delta H^o_f_{(CS_2)})+(3\times \Delta H^o_f_{(O_2)})]](/tpl/images/0163/6329/025c0.png)
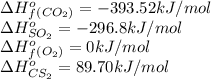
![\Delta H^o_{rxn}=[(1\times (-393.52))+(2\times (-296.8))]-[(1\times (89.70))+(3\times (0)]\\\\\Delta H^o_{rxn}=-1076.82kJ](/tpl/images/0163/6329/16879.png)



