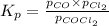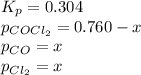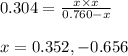Chemistry, 02.08.2019 23:20 meadowsoares7
The equilibrium constant kc for the decomposition of phosgene, cocl2, is 4.63x10^-3 at 527 c. calculate the equilibrium partial pressure of all the components, starting with pure phosgene at 0.760 atm

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 10:10, veronica022
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 11:00, snowprincess99447
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
The equilibrium constant kc for the decomposition of phosgene, cocl2, is 4.63x10^-3 at 527 c. calcul...
Questions in other subjects:



Chemistry, 02.01.2020 12:31

Chemistry, 02.01.2020 12:31

Biology, 02.01.2020 12:31


Mathematics, 02.01.2020 12:31

Mathematics, 02.01.2020 12:31


English, 02.01.2020 12:31

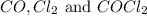 are 0.352 atm, 0.352 atm and 0.408 atm respectively.
are 0.352 atm, 0.352 atm and 0.408 atm respectively.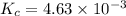
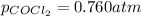
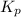 with
with 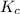 is given by the formula:
is given by the formula: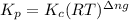
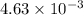
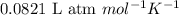
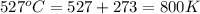
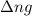 = change in number of moles of gas particles =
= change in number of moles of gas particles = 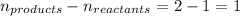
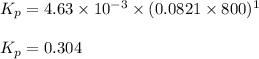
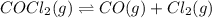
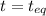 0.760-x x x
0.760-x x x