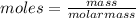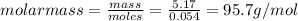Asample of an unknown compound is vaporized at 170 c. the gas produced has a volume of 1980 ml at a pressure of 1 atm, and it weighs 5.17 gr. assuming the gas behaves as an ideal gas under these conditions, calculate the molar mass of the compound. round your answer to 3 significant digits.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1


Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 02:00, xoxoadara13ox07ck
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
Asample of an unknown compound is vaporized at 170 c. the gas produced has a volume of 1980 ml at a...
Questions in other subjects:


Spanish, 04.02.2021 23:30



Business, 04.02.2021 23:30

Mathematics, 04.02.2021 23:30

Mathematics, 04.02.2021 23:30

Mathematics, 04.02.2021 23:30

Mathematics, 04.02.2021 23:30

Business, 04.02.2021 23:30