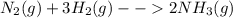Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 23.06.2019 00:00, glocurlsprinces
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3

Chemistry, 23.06.2019 02:30, hailee232
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1
You know the right answer?
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammon...
Questions in other subjects:


Computers and Technology, 26.06.2020 15:01



Mathematics, 26.06.2020 15:01



Mathematics, 26.06.2020 15:01

Mathematics, 26.06.2020 15:01

Chemistry, 26.06.2020 15:01