
Chemistry, 02.08.2019 20:30 LeoValdez5782
Suppose a layer of oil is on the top of a beaker of water. water in many oils is slightly soluble, so its concentration is so low that we can treat it as an ideal solute. 1) suppose that at 283 k, the equilibrium concentration is 9 × 10-4 water molecules per oil molecule, and it takes 2.208 × 10-20 j to transfer one water molecule into the oil. what is the equilibrium concentration at 293 k, assuming that nothing else changes

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:50, Amandachavez94
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 10:40, justicejesusfreak
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 22:30, lori90
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

Chemistry, 22.06.2019 23:00, hailey5campbelp7d1c0
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2
You know the right answer?
Suppose a layer of oil is on the top of a beaker of water. water in many oils is slightly soluble, s...
Questions in other subjects:


Health, 06.10.2019 21:30


Physics, 06.10.2019 21:30

Advanced Placement (AP), 06.10.2019 21:30

Mathematics, 06.10.2019 21:30




English, 06.10.2019 21:30

 J/molecule
J/molecule atoms.
atoms. J/mol
J/mol
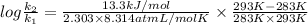
 = 0.08377
= 0.08377 = 1.213 =
= 1.213 = 
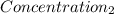 =
= 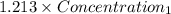

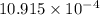 water molecules per oil molecule
water molecules per oil molecule

