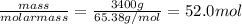Chemistry, 02.08.2019 19:10 abbeygrace13
Galvanized nails are iron nails that have been plated with zinc to prevent rusting. the relevant reaction is zn2+(aq)+2e−→zn(s) for a large batch of nails, a manufacturer needs to plate a total zinc mass of 3.40 kg on the surface to get adequate coverage. part a how many moles of zinc are in 3.40 kg of zinc? express your answer to three significant figures and include the appropriate units.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
Galvanized nails are iron nails that have been plated with zinc to prevent rusting. the relevant rea...
Questions in other subjects:


Computers and Technology, 21.07.2019 04:23



Physics, 21.07.2019 04:23


History, 21.07.2019 04:23

Mathematics, 21.07.2019 04:23

Social Studies, 21.07.2019 04:23

History, 21.07.2019 04:23