

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:50, kaitlyn2030
How do the energy differences between the higher energy levels of an atom compare with the energy difference between the lower energy level of the atom
Answers: 1

Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3


You know the right answer?
Agaseous hydrogen and carbon containing compound is decomposed and formed to contain 82.66% carbon a...
Questions in other subjects:

Mathematics, 28.08.2019 03:10


Biology, 28.08.2019 03:10

Mathematics, 28.08.2019 03:10

Mathematics, 28.08.2019 03:10

Mathematics, 28.08.2019 03:10


Mathematics, 28.08.2019 03:10

Mathematics, 28.08.2019 03:10

Mathematics, 28.08.2019 03:10


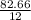

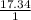
 = 2.5
= 2.5 .
.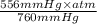
 equals 0.158 L.
equals 0.158 L.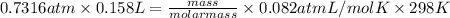
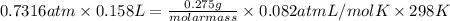
 = 29.
= 29. = 58
= 58 .
.

