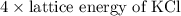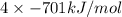Chemistry, 01.08.2019 06:10 izzyisawesome5232
Kclkcl has a lattice energy of −701 kj/mol.−701 kj/mol. consider a generic salt, abab , where a2+a2+ has the same radius as k+,k+, and b2−b2− has the same radius as xl−.xl−. estimate the lattice energy of the salt abab .

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 08:50, leah5981
Reacting masses1 calcium carbonate breaks down on heating to produce calcium oxide and carbondioxide gas. caco3 + cao + co2a student heats 15 g of calcium carbonate strongly in a crucible. relative atomic masses (a): ca = 40, c = 12, o = 16.calculate the mass of calcium oxide produced by this reaction.(5 marks)
Answers: 3

Chemistry, 23.06.2019 10:10, estebanmff
Solid tin exists in two forms: white and gray. for the transformation sn(s, white) → sn(s, gray) the enthalpy change is -2.1 kj/mol and the entropy change is -7.4 j/(mol*k). a. calculate the gibbs free energy change for the conversion of 1.00 mol white tin to gray tin at -30℃. b. will white tin convert spontaneously to gray tin at -30℃? c. at what temperature are white and gray tin thermodynamically equivalent at a pressure of 1 atm?
Answers: 3
You know the right answer?
Kclkcl has a lattice energy of −701 kj/mol.−701 kj/mol. consider a generic salt, abab , where a2+a2+...
Questions in other subjects:


Mathematics, 16.09.2021 08:10

Arts, 16.09.2021 08:10

Mathematics, 16.09.2021 08:10

Mathematics, 16.09.2021 08:10

History, 16.09.2021 08:10

Computers and Technology, 16.09.2021 08:10

Mathematics, 16.09.2021 08:20


Mathematics, 16.09.2021 08:20

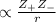
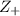 = +2, and
= +2, and  = -2
= -2