
Chemistry, 01.08.2019 05:20 idontcare2003
Suppose that 25.0 ml of 0.10 m ch3cooh (aq) is titrated with 0.10 m naoh (aq). what is the ph after the addition of 10.0 ml of 0.10 m naoh (aq)? (notice that the total volume of the solution changes with the addition of 10.0 ml of 0.10 m naoh)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:50, justabeachbum
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 10:00, winstonbendariovvygn
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 23.06.2019 00:00, baseball1525
Which item is most likely part of the safety contract
Answers: 1
You know the right answer?
Suppose that 25.0 ml of 0.10 m ch3cooh (aq) is titrated with 0.10 m naoh (aq). what is the ph after...
Questions in other subjects:

Chemistry, 12.07.2019 13:30

Biology, 12.07.2019 13:30

History, 12.07.2019 13:30

Mathematics, 12.07.2019 13:30

Mathematics, 12.07.2019 13:30

Mathematics, 12.07.2019 13:30

Mathematics, 12.07.2019 13:30

Biology, 12.07.2019 13:30


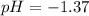
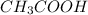 is titrated with 0.10 M NaOH(aq).
is titrated with 0.10 M NaOH(aq).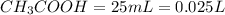
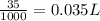
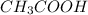 =0.10 M
=0.10 M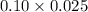 =0.0025 moles
=0.0025 moles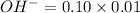 =0.001mole
=0.001mole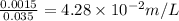
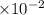 ]=-log4.28+2 log 10=-0.631+2
]=-log4.28+2 log 10=-0.631+2

