Chemistry, 01.08.2019 04:20 arodriguez395
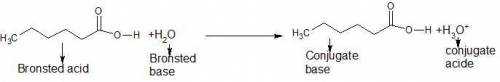
One of the substances that give wet goats and dirty gym socks their characteristic odors is hexanoic acid, ch3ch2ch2ch2ch2co2h, which is a monoprotic weak acid. write the formula for the conjugate base of this acid. write the equation for the reaction between this acid and water, and indicate the brønstedlowry acid and base for the forward reaction. (the acidic hydrogen atom is on the right side of the formula.)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, robert7248
What is the cellular process that releases the energy stored in food molecules
Answers: 3


You know the right answer?
One of the substances that give wet goats and dirty gym socks their characteristic odors is hexanoic...
Questions in other subjects:


Mathematics, 13.04.2020 21:21



Mathematics, 13.04.2020 21:21