

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:20, zymikaa00
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 21.06.2019 22:30, kkruvc
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 20:30, jaydenbrock
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 23.06.2019 00:30, evelynalper08
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
Consider the following equation: n2o4(g) ⇄ 2 no2(g) kc = 5.8 × 10-3 if the initial concentration of...
Questions in other subjects:

Health, 20.02.2020 01:57


Biology, 20.02.2020 01:57



Computers and Technology, 20.02.2020 01:57

Physics, 20.02.2020 01:57



Social Studies, 20.02.2020 01:58

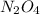 at equilibrium is, 0.03977 M
at equilibrium is, 0.03977 M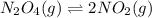
![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0156/8648/271f5.png)
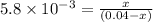
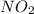 at equilibrium = 2x M = 2 × 0.00023 = 0.00046 M
at equilibrium = 2x M = 2 × 0.00023 = 0.00046 M

