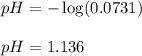Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2


Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
In a titration of 47.41 ml of 0.3764 m ammonia with 0.3838 m aqueous nitric acid, what is the ph of...
Questions in other subjects:

Spanish, 25.01.2021 15:10


Biology, 25.01.2021 15:10

Mathematics, 25.01.2021 15:10






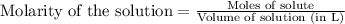
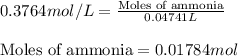
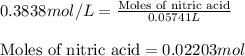
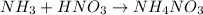
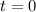 0.0178 0.022
0.0178 0.022![pH=-\log[H^+]](/tpl/images/0156/8638/cf945.png)
![[H^+]=\frac{0.0042mol}{0.05741L}=0.0731M](/tpl/images/0156/8638/dda8c.png)