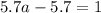Answers: 2


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 02:00, jacckiie5176
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 04:31, diamondscott9297
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1
You know the right answer?
For the chemical equation so2(g)+no2(g)↽−−⇀so3(g)+no(g) the equilibrium constant at a certain temper...
Questions in other subjects:

Mathematics, 24.11.2019 09:31

Mathematics, 24.11.2019 09:31


Mathematics, 24.11.2019 09:31



History, 24.11.2019 09:31



![Kc=\frac{[NO][SO_{3}]}{[NO_{2}][SO_{2}]}](/tpl/images/0156/8615/e939f.png)
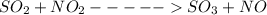
![Kc=3.80 = \frac{[1][1]}{[a-1][1.5]}](/tpl/images/0156/8615/9a395.png)