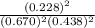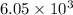Chemistry, 01.08.2019 03:20 makeithappen60
At 250°c an equilibrium mixture of sbcl3(g), cl2(g), and sbcl5(g) has the partial pressures 0.670 bar, 0.438 bar, and 0.228 bar, respectively. calculate the new equilibrium pressures if the volume of the reaction vessel is doubled.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
At 250°c an equilibrium mixture of sbcl3(g), cl2(g), and sbcl5(g) has the partial pressures 0.670 ba...
Questions in other subjects:

Chemistry, 17.01.2021 09:00


Business, 17.01.2021 09:00


Law, 17.01.2021 09:00




English, 17.01.2021 09:00

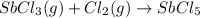
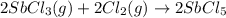
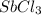 is 0.670 bar,
is 0.670 bar, 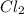 is 0.438 bar and
is 0.438 bar and 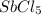 is 0.228 bar.
is 0.228 bar. ![K_{p} = \frac{[P_{SbCl_{5}}]^{2}}{[P_{SbCl_{3}}]^{2}[P_{Cl_{2}}]^{2}}](/tpl/images/0156/7126/bcfbb.png)