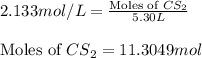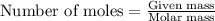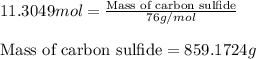Carbon disulfide is prepared by heating sulfur and charcoal. the chemical equation is s2(g)+c(s)↽−−⇀cs2(=9.40 at 900 k how many grams of cs2(g) can be prepared by heating 12.5 mol s2(g) with excess carbon in a 5.30 l reaction vessel held at 900 k until equilibrium is attained?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 20:10, sarahalexa19
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 00:50, maddysmall32
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1

Chemistry, 23.06.2019 10:00, eyeneedalife
Abike ride event is 30 miles. a first aid tent is put at the 3/4 mark of the course. how many miles from the starting point is the first aid tent?
Answers: 1
You know the right answer?
Carbon disulfide is prepared by heating sulfur and charcoal. the chemical equation is s2(g)+c(s)↽−−⇀...
Questions in other subjects:

Social Studies, 12.02.2021 21:50

Social Studies, 12.02.2021 21:50



Physics, 12.02.2021 21:50

Chemistry, 12.02.2021 21:50



Chemistry, 12.02.2021 21:50

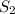 , we use the equation:
, we use the equation: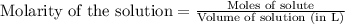 .....(1)
.....(1)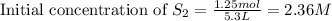
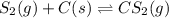
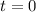 2.36
2.36 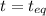 2.36 - x x
2.36 - x x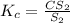

![[CS_2]=x](/tpl/images/0156/7037/d556c.png)
![[S_2]=2.36-x](/tpl/images/0156/7037/9691b.png)
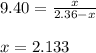
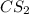 = 2.133 M
= 2.133 M