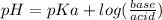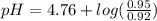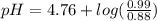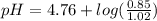Calculate the ph of 1.00 l of the buffer 0.95 m ch3coona/0.92 m ch3cooh before and after the addition of the following species. (assume there is no change in volume.) (a) ph of starting buffer: (b) ph after addition of 0.040 mol naoh: (c) ph after further addition of 0.100 mol hcl:

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, taysomoneyyy
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 21.06.2019 22:30, pinkycupcakes3oxbqhx
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 04:00, BaileyElizabethRay
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3
You know the right answer?
Calculate the ph of 1.00 l of the buffer 0.95 m ch3coona/0.92 m ch3cooh before and after the additio...
Questions in other subjects:




Computers and Technology, 05.05.2020 12:17

Physics, 05.05.2020 12:17



Social Studies, 05.05.2020 12:17


Mathematics, 05.05.2020 12:17