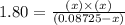Chemistry, 01.08.2019 02:20 tinapersaud1587
Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(=1.80 at 250 ∘c a 0.349 mol sample of pcl5(g) is injected into an empty 4.00 l reaction vessel held at 250 ∘c. calculate the concentrations of pcl5(g) and pcl3(g) at equilibrium.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:00, montgomerykarloxc24x
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 23.06.2019 03:30, alecnewman2002
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

Chemistry, 23.06.2019 07:00, jstyopin
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1
You know the right answer?
Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(=1.80...
Questions in other subjects:



Mathematics, 11.07.2019 18:30

Mathematics, 11.07.2019 18:30

Biology, 11.07.2019 18:30

Mathematics, 11.07.2019 18:30

Geography, 11.07.2019 18:30



 and
and  at equilibrium are, 0.0834 M and 0.00385 M
at equilibrium are, 0.0834 M and 0.00385 M

![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0156/5529/73fe0.png)