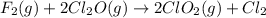Chemistry, 01.08.2019 01:30 unknown9263
The following initial rate data apply to the reaction f2(g) + 2cl2o(g) ® 2clo2(g) + cl2(g).which of the following is the rate law (rate equation) for this reaction? rate = k[f2][cl2o]rate = k[f2]2[cl2o]2rate = k[f2][cl2o]2rate = k[f2]2[cl2o]4rate = k[f2]2[cl2o]

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2
You know the right answer?
The following initial rate data apply to the reaction f2(g) + 2cl2o(g) ® 2clo2(g) + cl2(g).which of...
Questions in other subjects:

Social Studies, 28.05.2021 08:50

Mathematics, 28.05.2021 08:50


Biology, 28.05.2021 08:50

Mathematics, 28.05.2021 08:50


Mathematics, 28.05.2021 08:50

Mathematics, 28.05.2021 09:00

English, 28.05.2021 09:00

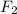 ]
] ![[Cl_{2}O]^{2}](/tpl/images/0156/4052/463f7.png)