
Chemistry, 01.08.2019 01:20 christophergaudette0
Consider the following reactions and their respective equilibrium constants: no(g)+12br2(g)⇌nobr(g)kp=5.3 2no(g)⇌n2(g)+o2(g)kp=2.1×1030 use these reactions and their equilibrium constants to predict the equilibrium constant for the following reaction: n2(g)+o2(g)+br2(g)⇌2nobr(g)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 19:00, cindyroxana229
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
Consider the following reactions and their respective equilibrium constants: no(g)+12br2(g)⇌nobr(g)...
Questions in other subjects:


Mathematics, 10.12.2021 17:30


Physics, 10.12.2021 17:30

English, 10.12.2021 17:30

Biology, 10.12.2021 17:30

Chemistry, 10.12.2021 17:30

Mathematics, 10.12.2021 17:30



 ;
; 
 ;
; 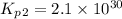
 ;
; 






