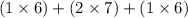Be sure to answer all parts. dichlorine heptaoxide, cl2o7, can be viewed as two clo4 groups sharing an o atom. draw a lewis structure for cl2o7 with the lowest formal charges and predict any deviation from the ideal for the cl―o―cl bond angle. be sure to include lone-pair electrons.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1

Chemistry, 22.06.2019 21:00, andrethisman88
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
Be sure to answer all parts. dichlorine heptaoxide, cl2o7, can be viewed as two clo4 groups sharing...
Questions in other subjects:




History, 16.10.2020 16:01

Chemistry, 16.10.2020 16:01


Mathematics, 16.10.2020 16:01



Mathematics, 16.10.2020 16:01

 ) can also be written as
) can also be written as  as one oxygen atom will be at the center and there will be two terminal
as one oxygen atom will be at the center and there will be two terminal  groups.
groups.