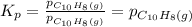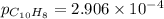Asample of solid naphthalene is introduced into an evacuated flask. use the data below to calculate the equilibrium vapor pressure of naphthalene (c10h8) in the flask at 35°c. hf (25c) gf (25c) c10h8(s) 78.5 kj/mol 201.6 kj/mol c10h8(g) 150.6 kj/mol 224.1 kj/mol

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, mutoni55
Consider three unlabeled bottles, each contain small pieces of one of the following metals. - magnesium - sodium - silver the following reagents are used for identifying the metals. - pure water - a solution of 1.0 molar hcl - a solution of concentrated hno3 (a) which metal can be easily identified because it is much softer than the other two? describe a chemical test that distinguishes this metal from the other two, using only one of the reagents above. write a balanced chemical equation for the reaction that occurs. (b) one of the other two metals reacts readily with the hcl solution. identify the metal and write the balanced chemical equation for the reaction that occurs when this metal is added to the hcl solution. use the table of standard reduction potentials (attached) to account for the fact that this metal reacts with hcl while the other does not. (c) the one remaining metal reacts with the concentrated hno3 solution. write a balanced chemical equation for the reaction that occurs. (d) the solution obtained in (c) is diluted and a few drops of 1 m hcl is added. describe what would be observed. write a balanced chemical equation for the reaction that occurs.
Answers: 2

Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 19:30, xxaurorabluexx
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 23.06.2019 01:10, minasotpen1253
Volume is a measurement of how fast particles of a substance are moving
Answers: 3
You know the right answer?
Asample of solid naphthalene is introduced into an evacuated flask. use the data below to calculate...
Questions in other subjects:


English, 16.04.2020 22:18

History, 16.04.2020 22:18


English, 16.04.2020 22:18

Social Studies, 16.04.2020 22:18




 atm.
atm.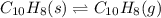
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0156/3886/45485.png)
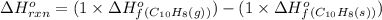
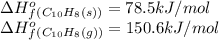
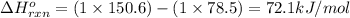
![\Delta G^o_{rxn}=\sum [n\times \Delta G^o_f(product)]-\sum [n\times \Delta G^o_f(reactant)]](/tpl/images/0156/3886/b00b4.png)
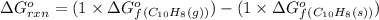
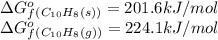
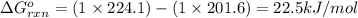
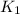 (at 25°C) for given value of Gibbs free energy, we use the relation:
(at 25°C) for given value of Gibbs free energy, we use the relation: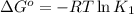
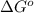 = Gibbs free energy = 22.5 kJ/mol = 22500 J/mol (Conversion factor: 1kJ = 1000J)
= Gibbs free energy = 22.5 kJ/mol = 22500 J/mol (Conversion factor: 1kJ = 1000J)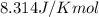
![25^oC=[273+25]K=298K](/tpl/images/0156/3886/0e82f.png)
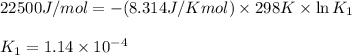
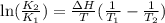
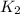 = Equilibrium constant at 35°C = ?
= Equilibrium constant at 35°C = ?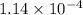
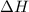 = Enthalpy change of the reaction = 72.1 kJ/mol = 72100 J
= Enthalpy change of the reaction = 72.1 kJ/mol = 72100 J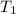 = Initial temperature =
= Initial temperature = 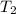 = Final temperature =
= Final temperature = ![35^oC=[273+35]K=308K](/tpl/images/0156/3886/b2088.png)
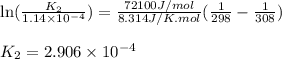
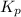 , which is:
, which is: