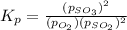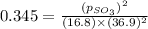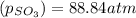Chemistry, 31.07.2019 23:10 yournerdybirdyp43oi3
At 900.0 k, the equilibrium constant (kp) for the following reaction is 0.345. 2so2 + o2 (g) →2 so3 (g) at equilibrium, the partial pressure of so2 is 36.9 atm and that of o2 is 16.8 atm. the partial pressure of so3 is atm.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, 2024cynthiatercero
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2


Chemistry, 22.06.2019 18:50, cj31150631
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
At 900.0 k, the equilibrium constant (kp) for the following reaction is 0.345. 2so2 + o2 (g) →2 so3...
Questions in other subjects:

Mathematics, 09.05.2021 14:00

Mathematics, 09.05.2021 14:00



Business, 09.05.2021 14:00

Mathematics, 09.05.2021 14:00

Business, 09.05.2021 14:00


English, 09.05.2021 14:00

Mathematics, 09.05.2021 14:00

 is, 88.84 atm
is, 88.84 atm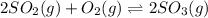
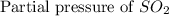 = 36.9 atm
= 36.9 atm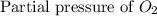 = 16.8 atm
= 16.8 atm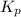 will be,
will be,