The decomposition of hydrogen peroxide follows first order kinetics and has a rate constant of 2.54 x 10-4 s-1 at a certain temperature. if the concentration of hydrogen peroxide is 0.321 m after 855 s , what was the initial concentration of hydrogen peroxide at this temperature? 0.258 m0.399 m0.538 m0.677 m1.48 m

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, happy121906
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 21:00, taylorlanehart
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 22.06.2019 23:00, liv467
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 23.06.2019 00:20, destromero
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
The decomposition of hydrogen peroxide follows first order kinetics and has a rate constant of 2.54...
Questions in other subjects:

History, 04.11.2019 06:31

Engineering, 04.11.2019 06:31


History, 04.11.2019 06:31

Biology, 04.11.2019 06:31

History, 04.11.2019 06:31



Biology, 04.11.2019 06:31

English, 04.11.2019 06:31

![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0155/7548/f1041.png)
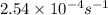
![[A_o]](/tpl/images/0155/7548/dc622.png) = initial amount of the reactant = ?
= initial amount of the reactant = ?![2.54\times 10^{-4}s^{-1}=\frac{2.303}{855s}\log \frac{[A_o]}{0.321}](/tpl/images/0155/7548/e899e.png)
![[A_o]=0.399M](/tpl/images/0155/7548/ee654.png)