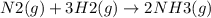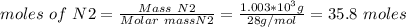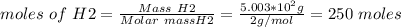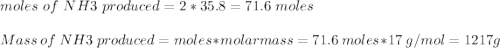Chemistry, 31.07.2019 19:10 zdwilliams1308
Ammonia is produced from the reaction of nitrogen and hydrogen according to the following balanced equation: n2 1 g 2 1 3h2 1 g 2 h 2nh3 1 g 2
a. what is the maximum mass of ammonia that can be produced from a mixture of 1.00 3 103 g n2 and 5.00 3 102 g h2

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:50, jordan5778
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2
You know the right answer?
Ammonia is produced from the reaction of nitrogen and hydrogen according to the following balanced e...
Questions in other subjects:










Computers and Technology, 22.08.2020 03:01