
Chemistry, 31.07.2019 19:10 brianlykid3042
Calculate the percent ionization of formic acid (hco2h) in a solution that is 0.125 m in formic acid. the ka of formic acid is 1.77 ⋅ 10-4. calculate the percent ionization of formic acid (hco2h) in a solution that is 0.125 m in formic acid. the ka of formic acid is 1.77 10-4. 0.859 0.0180 3.79 2.25 ⋅ 10-5 6.94

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, sammysosa121832
The ph of carrots are 5.0 how it is classified a. acidic b. basic c. indicator d. neutral
Answers: 2


Chemistry, 21.06.2019 22:00, pollywallythecat
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

You know the right answer?
Calculate the percent ionization of formic acid (hco2h) in a solution that is 0.125 m in formic acid...
Questions in other subjects:

Physics, 29.11.2020 14:00

Mathematics, 29.11.2020 14:00


Chemistry, 29.11.2020 14:00

Mathematics, 29.11.2020 14:00

English, 29.11.2020 14:00


Social Studies, 29.11.2020 14:00

Chemistry, 29.11.2020 14:00

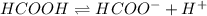
![\frac{[HCOO^{-}][H^{+}]}{[HCOOH]}=K_{a}(HCOOCH)](/tpl/images/0155/4252/45db7.png)
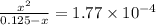
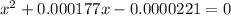

 M
M![[H^{+}]=4.61\times 10^{-3}M](/tpl/images/0155/4252/38c16.png)
![\frac{[H^{+}]}{initial concentration of HCOOH}\times 100](/tpl/images/0155/4252/17262.png) =
= 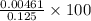 = 3.69%
= 3.69%

