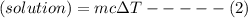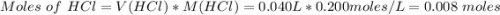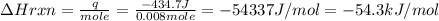When 40.0 ml of 0.200 m hcl at 21.5°c is added to 40.0 ml of 0.200 m naoh also at 21.5°c in a coffee-cup calorimeter, the temperature of the resulting solution rises to 22.8°c. assume that the volumes are additive, the specific heat of the solution is 4.18 jg -1°c -1 and that the density of the solution is 1.00 g ml -1 calculate the enthalpy change, δh in kj for the reaction:

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 07:20, rscvsdfsrysas1857
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 09:00, triddi666
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1
You know the right answer?
When 40.0 ml of 0.200 m hcl at 21.5°c is added to 40.0 ml of 0.200 m naoh also at 21.5°c in a coffee...
Questions in other subjects:







Mathematics, 23.06.2019 16:00