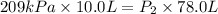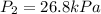Chemistry, 31.07.2019 17:20 houtchhaytang
Acylinder is filled with 10.0l of gas and a piston is put into it. the initial pressure of the gas is measured to be 209.kpa. the piston is now pulled up, expanding the gas, until the gas has a final volume of 78.0l. calculate the final pressure of the gas. be sure your answer has the correct number of significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2


Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Acylinder is filled with 10.0l of gas and a piston is put into it. the initial pressure of the gas i...
Questions in other subjects:



Mathematics, 01.02.2021 06:00



Mathematics, 01.02.2021 06:00

Health, 01.02.2021 06:00


Arts, 01.02.2021 06:00

Chemistry, 01.02.2021 06:00

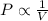
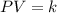
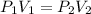
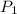 = initial pressure of the gas = 209 kPa
= initial pressure of the gas = 209 kPa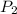 = final pressure of the gas = ?
= final pressure of the gas = ?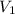 = initial volume of the gas = 10.0 L
= initial volume of the gas = 10.0 L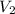 = final volume of the gas = 78.0 L
= final volume of the gas = 78.0 L