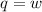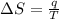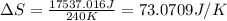Suppose 4.00 mol of an ideal gas undergoes a reversible isothermal expansion from volume v1 to volume v2 = 9v1 at temperature t = 240 k. find (a) the work done by the gas and (b) the entropy change of the gas. (c) if the expansion is reversible and adiabatic instead of isothermal, what is the entropy change of the gas?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 10:00, isaiahromero15
The image shows the process of which is used in nuclear power plants. photo attached
Answers: 1

Chemistry, 23.06.2019 12:30, amendes11
How does a nuclear reactor produce electricity? a. high-energy gamma rays are converted by a generator into electricity. b. the heat from the reaction turns water to steam, which runs a generator. c. the reaction produces a stream of electrons that flow through wires and into batteries. d. the heat released from the reaction is used to burn coal or gas to produce electricity. e. control rods absorb the neutrons emitted and release a stream of electrons as electricity.
Answers: 1

Chemistry, 23.06.2019 13:30, sophie5988
Where are electrons with the lowest energy found? in the nucleus farthest from the nucleus outside the atom closest to the nucleus
Answers: 1
You know the right answer?
Suppose 4.00 mol of an ideal gas undergoes a reversible isothermal expansion from volume v1 to volum...
Questions in other subjects:

English, 10.03.2020 04:11





Computers and Technology, 10.03.2020 04:12

Chemistry, 10.03.2020 04:12



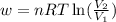
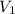 = initial volume of gas =
= initial volume of gas = 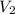 = final volume of gas =
= final volume of gas = 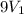
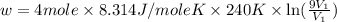
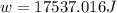
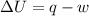
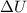 = internal energy
= internal energy